NEWS and OPINIONS
Axiom Space establishes Winston-Salem presence in the Regenerative Medicine Hub
WINSTON-SALEM, NC, April 18, 2022 – Regenerative medicine manufacturing in space is the next frontier and will be possible due to a new three-way partnership between Axiom Space, which is building the world’s first commercial space station, the RegenMed Development...
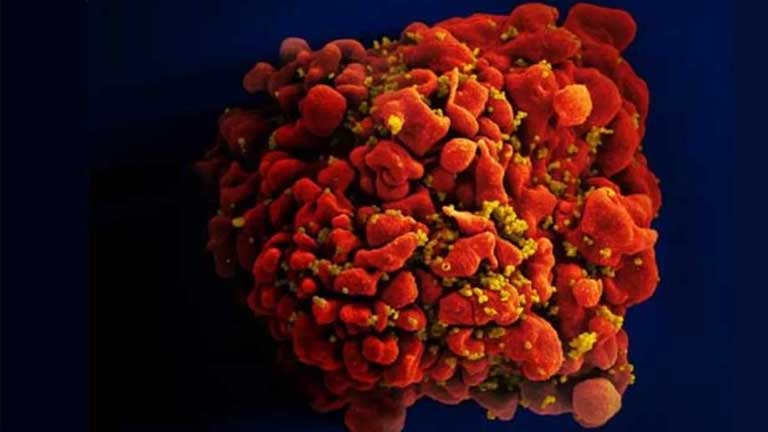
NIH launches clinical trial of three mRNA HIV vaccines
Image caption: scanning electron micrograph of an HIV-infected H9 T cell. Phase 1 study is among first to examine mRNA technology for HIV The National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, has launched a Phase...
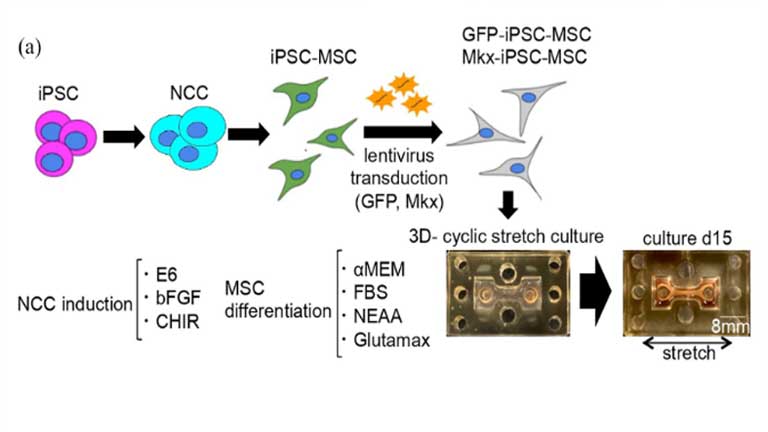
Generation of a tendon-like tissue from human iPS cells
Published in Sage Journals - Link to article Tendons and ligaments are essential connective tissues that connect the muscle and bone. Their recovery from injuries is known to be poor, highlighting the crucial need for an effective therapy. A few reports have...

New technique can upregulate specific genes without permanently altering the genome
Reviewed by Emily Henderson, B.Sc - news-medical.net - link to article By combining CRISPR technology with a protein designed with artificial intelligence, it is possible to awaken individual dormant genes by disabling the chemical "off switches" that silence them....

Process for Extending the Longevity of Stem Cells Described
Original story from University of California, Santa Barbara People are having children later than ever before. The average age of new parents in the United States has been rising for at least the past half century. But time is tough on our bodies and our reproductive...

Can We Resurrect Extinct Species? Scientists Put Jurassic Park to the Test
By Shelly Fan - Link to article De-extinction grabbed our imagination in the 90s with Jurassic Park. Scientists have since asked: how possible is it? According to a new study, nearly impossible. But wait—it’s not all bad news. While bringing back a faithful copy of an...

Weill Scientists Explain Breakthrough in Potential HIV Cure
By Tiffany Adjei-Opong - Cornell Daily Sun - Link to Article Four years ago, a team of research physicians at Weill Cornell Medicine began treatment for an HIV patient, in the hopes of finding a cure. This February marked 14 months since the patient was free of the...

2 new molecules help destroy leukemia cells by targeting protein key to tumor ‘seeds’
By Kyle LaHucik - Fiercebiotech.com Relapses in acute myeloid leukemia (AML), the white blood cell cancer that affects blood and bone marrow, remain an obstacle despite new treatments, as the average five-year survival rate sits at 25%. Now, researchers say they’ve...
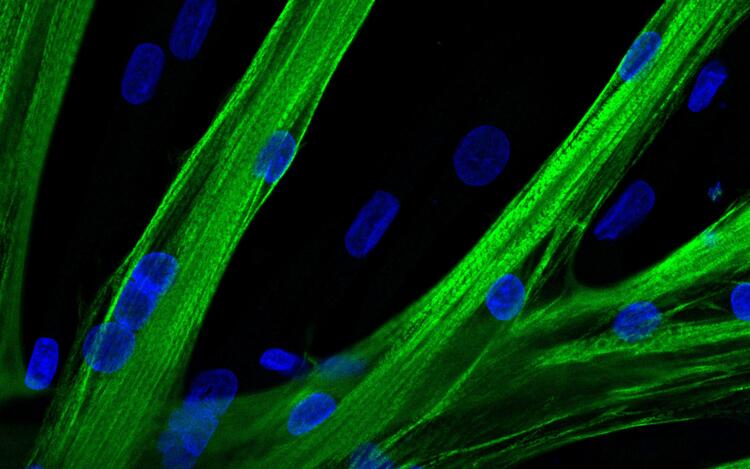
Using mRNA delivery to improve muscle strength
By Anke Brodmerkel - MDC website - Link to article Berlin Germany, March 14 2022 - Mutations that lead to muscle atrophy can be repaired with the gene editor CRISPR-Cas9. A team led by ECRC researcher Helena Escobar has now introduced the tool into human muscle stem...
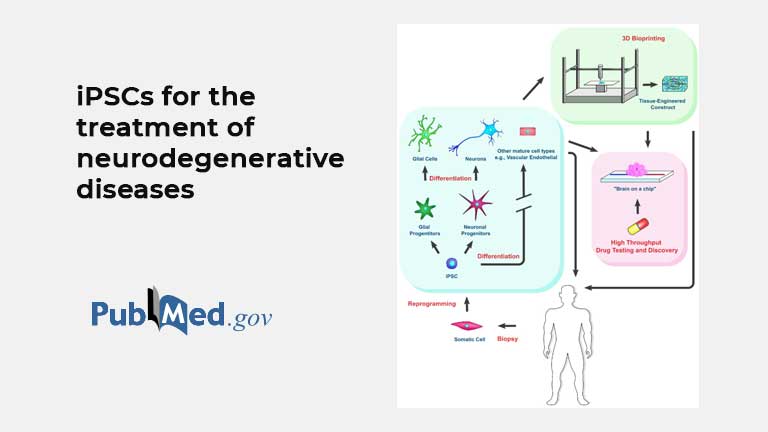
Induced Pluripotent Stem Cells for Treatment of Alzheimer’s and Parkinson’s Diseases
https://pubmed.ncbi.nlm.nih.gov/35203418/ David A Yefroyev 1 , Sha Jin 1 Affiliations PMID: 35203418 PMCID: PMC8869146 DOI: 10.3390/biomedicines10020208 Free PMC article Abstract Neurodegenerative diseases are a group of debilitating pathologies in which neuronal...

Breaking News! 2022 Virtual World Stem Cell Summit and Regenerative Medicine Essentials Course to be Held June 6-11
The Regenerative Medicine Foundation, in partnership with the Wake Forest Institute for Regenerative Medicine, is proud to announce the 2022 VIRTUAL WORLD STEM CELL SUMMIT & REGENERATIVE MEDICINE ESSENTIALS COURSE, June 6 - 11, 2022. This famed six-day interactive...
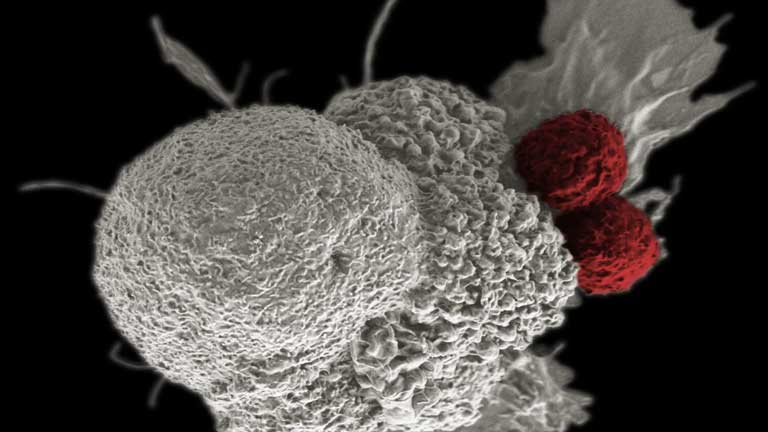
A ‘living’ cancer drug helped two patients stay disease-free for a decade
image Caption: two red cells attack a white cell, the cells have been pseudo-colored - Two T-cells (red) attack an oral squamous cancer cell (white)—a fight that's part of the natural immune response. Clinical researchers are developing a new type of therapy that...
