By By Erin Digitale, Stanford Medicine – SciTechDaily –
A Phase 1 clinical trial has shown that an antibody developed at Stanford Medicine can prepare patients for stem cell transplantation while avoiding toxic side effects.
A phase 1 clinical trial has demonstrated that an antibody treatment created at Stanford Medicine can safely prepare patients for stem cell transplants without the need for busulfan chemotherapy or radiation, both of which are highly toxic.
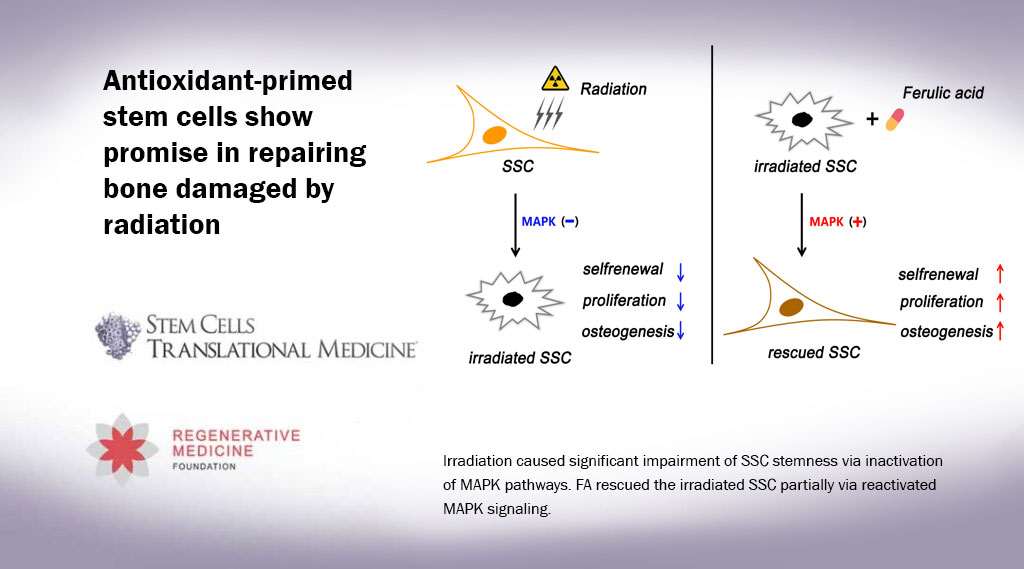
The trial focused on children with Fanconi anemia, a rare genetic disorder that makes conventional stem cell transplantation especially dangerous. Although the study tested this new protocol in Fanconi patients, the researchers believe it could also benefit people with other genetic conditions that require transplants.