By Emily Mullin – WIRED –
Florida is the latest state to sidestep the authority of the Food and Drug Administration by allowing patients to access certain stem-cell treatments that have not been rigorously evaluated and approved.
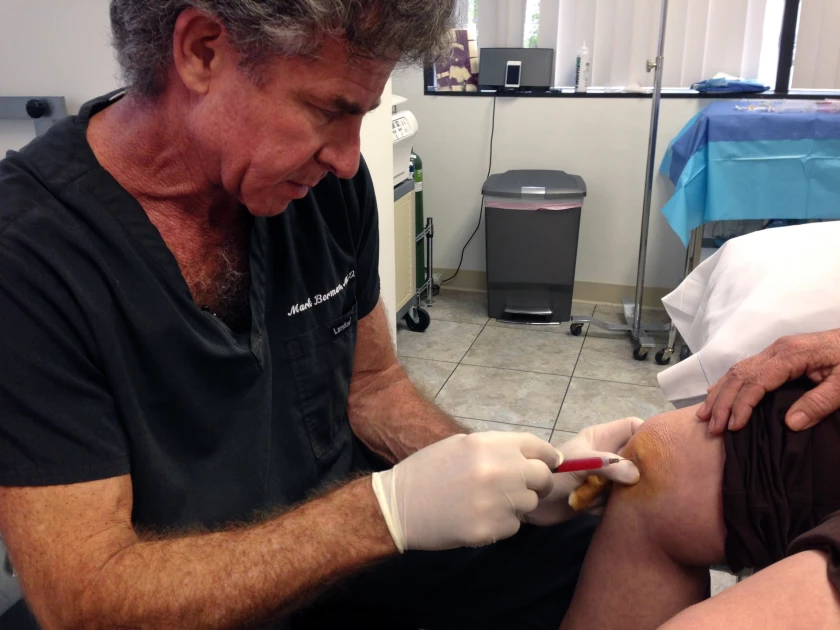
Under a new law that went into effect July 1, doctors in Florida can administer unapproved stem-cell therapies for wound care, pain management, or orthopedic purposes. The law comes amid growing support for medical freedom in the United States, an idea espoused by Health and Human Services secretary Robert F. Kennedy Jr., and it could prompt other states to follow. Supporters say the law helps protect patients, while critics argue it opens the door for physical and financial harm.
“There is interest in various states in enabling the sale of stem-cell products that have not been approved by the FDA,” says Leigh Turner, a bioethicist and professor of health, society, and behavior at the University of California, Irvine, who has been tracking the stem-cell industry. “I think we’re going to see more of that.”