By Japan Times
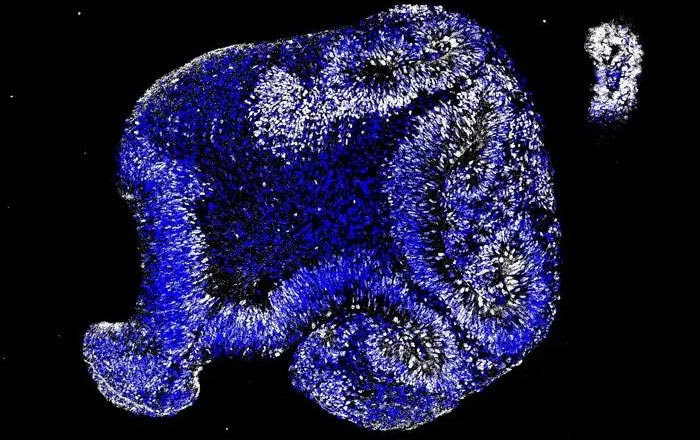
A Japanese medical institution, in a first for the country, will seek government approval for using embryonic stem cells as a regenerative medicine product to treat babies with a severe liver ailment, sources close to the matter said Monday.
The step toward commercialization of the treatment comes after the National Center for Child Health and Development confirmed its safety and efficacy in a clinical trial between October 2019 and December 2021.