By Rebekah McBride – University of Wisconsin–Madison
Lab-grown human heart cells provide a powerful tool to understand and potentially treat heart disease. However, the methods to produce human heart cells from pluripotent stem cells are not optimal. Fortunately, a new study out of the University of Wisconsin–Madison Stem Cell & Regenerative Medicine Center is providing key insight that will aid researchers in growing cardiac cells from stem cells.
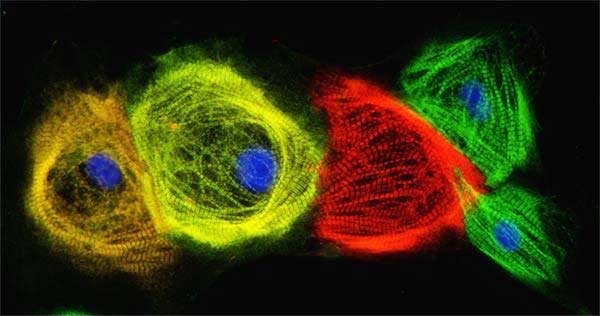
The research, published recently in eLife, investigates the role of extracellular matrix (ECM) proteins in the generation of heart cells derived from human pluripotent stem cells (hPSCs). The ECM fills the space between cells, providing structural support and regulating formation of tissues and organs. With a better understanding of ECM and its impact on heart development, researchers will be able to more effectively develop heart muscle cells, called cardiomyocytes, that could be useful for cardiac repair, regeneration and cell therapy.
“How the ECM impacts the generation of hPSC-cardiomyocytes has been largely overlooked,” says Jianhua Zhang, a senior scientist at the Stem Cell and Regenerative Medicine Center who led the study along with Tim Kamp, who serves as director of the center and Cellular and Molecular Arrhythmia Research Program. “The better we understand how the soluble factors as well as the ECM proteins work in the cell culture and differentiation, the closer we get to our goals.”
Researchers like Zhang have been looking to improve the differentiation of hPSCs into cardiomyocytes, or the ability to take hPSCs, which can self-renew indefinitely in culture while maintaining the ability to become almost any cell type in the human body and turn them into heart muscle cells. To investigate the role of the ECM in promoting this cardiac differentiation of hPSCs, Zhang tested a variety of proteins to see how they impacted stem cell growth and differentiation — specifically, ECM proteins including laminin-111, laminin-521, fibronectin and collagen.
“Our study showed ECM proteins play significant roles in the hPSC adhesion, growth, and cardiac differentiation. And fibronectin plays an essential role and is indispensable in hPSC cardiac differentiation,” says Zhang. “By understanding the roles of ECM, this study will help to develop more robust methods and protocols for generation of hPSC-CMs. Furthermore, this study not only helps in the field for cardiac differentiation, but also other lineage differentiation as well.”
While the new study provides important insight into heart cell development, it is built upon a 2012 study Zhang led which looked at the most efficient way to develop cardiac differentiation of stem cells.
“This study is actually a follow-up paper to the Matrix Sandwich Method that we developed for efficient cardiac differentiation of hPSCs,” Zhang says. “In order to culture the stem cells, we needed to have an ECM layer on the bottom of the plate. Otherwise, the stem cells would not attach to the plate. We would then add another layer of ECM on top of the growing stem cells, and we found that this helped promote the most effective differentiation.”
While it was clear that this layering, or sandwich, method more efficiently and reproducibly differentiated hPSC-cardiomyocytes, researchers did not fully understand why. The new study explains why the ECM layers are crucial and identifies fibronectin as a key ECM protein in the development of hPSC-cardiomyocytes.
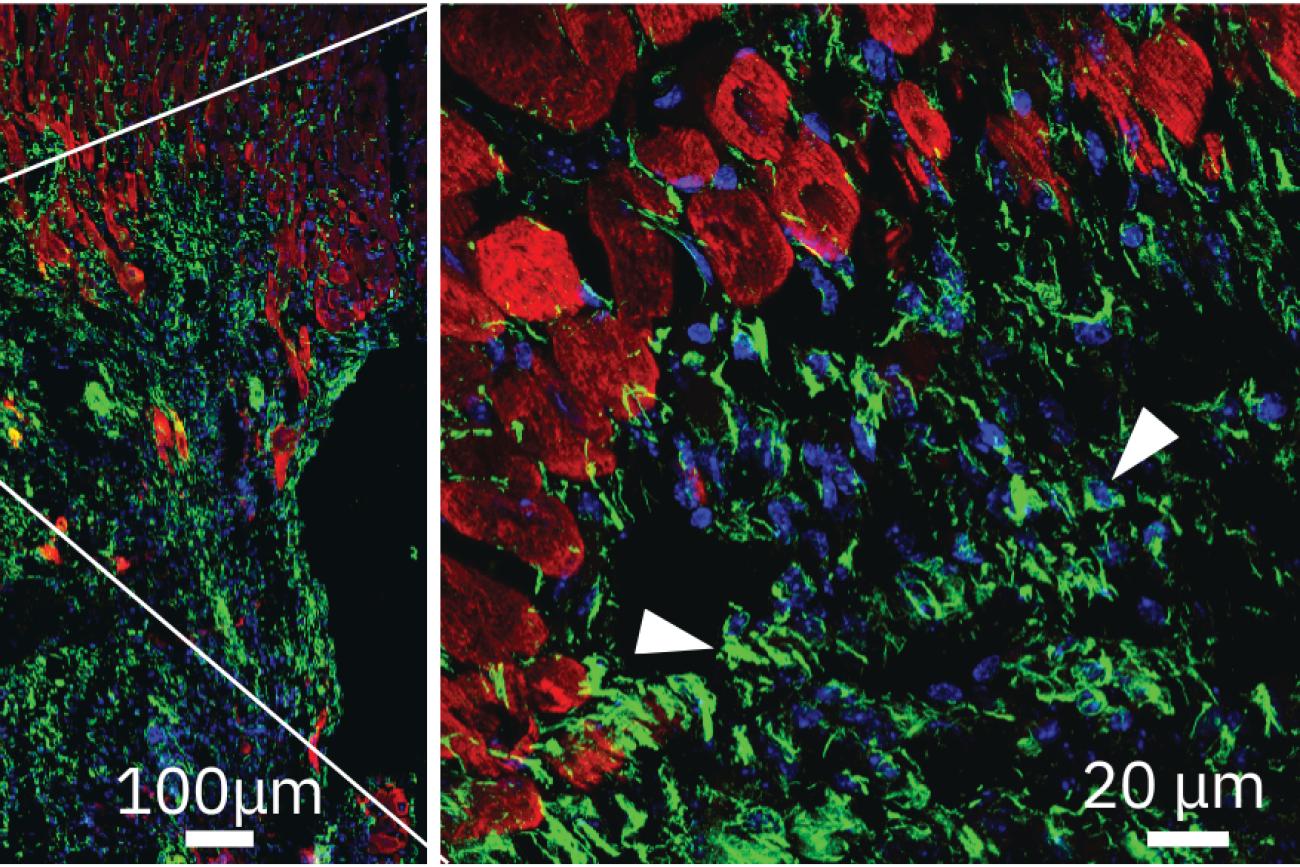
“The most exciting part of this study is now I understand why the Matrix Sandwich Method worked. We were able to identify the fibronectin and its integrin receptors as well as the downstream signaling pathways in this study,” Zhang explains. “With a better understanding of ECM’s roles in stem cell growth and cardiac differentiation, we now hope to investigate the roles of fibronectin and other ECM proteins in promoting the hPSC-cardiomyocytes transplantation for cell therapy.”
The next step could help researchers realize the full potential of using hPSC-cardiomyocytes for disease modeling, drug screening, cardiac regeneration and cell therapy. This is very meaningful to Zhang, who began working in cardiovascular research more than 16 years ago.
“I became interested in stem cell and heart research when I began working with the stem cells and saw them turning into heart cells beating in a cell culture dish under a microscope,” Zhang says. “It was amazing. I’ve become more and more dedicated to this research, and I can really see the potential of using the stem cell technologies to cure disease and improve our health.”