From the Lieber and Kiem Labs, Hematologic Malignancies Program, Cancer Consortium.
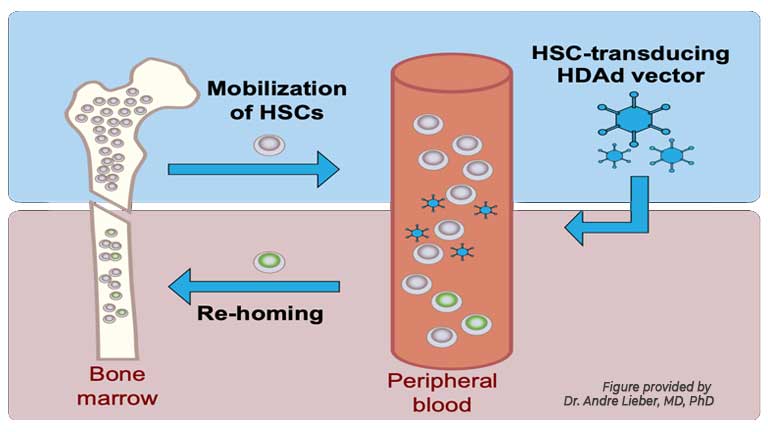
Nestled in the pillowy, marrow-filled interior of your bones is a special group of cells called hematopoietic stem cells (HSCs) that are responsible for generating and re-generating your red and white blood cells throughout life. Unfortunately, in hereditary diseases, HSCs that carry mutations just like any other cell, pass them along to their progeny. For instance, hemoglobinopathies, mutations that affect the hemoglobin protein that is responsible for binding oxygen in red blood cells, arise from germline mutations in HSCs and include diseases such as thalassemia and sickle cell anemia. For decades, therapies for these diseases have been supportive, not curative, including either red blood cell transfusion from an unaffected donor or HSC transplant. Transfusions have limited utility as red blood cells only live for 100-120 days. However, researchers have pivoted recently towards gene therapy approaches that reprogram a portion of a patient’s own HSCs. This therapy relies on retrieving HSCs from the bone marrow, introducing a corrected copy of the affected gene, then dosing the patient with high dose chemotherapy to make room in their bone marrow for engraftment of the reprogrammed cells. It’s a risky, high-cost, and technically complex process – the perfect target for technological innovation. A collaborative study published this month in Molecular Therapy: Methods & Clinical Development by the Lieber and Kiem lab groups describes a technique that would allow clinicians to reprogram HSCs inside patients’ bodies without the need for high-dose chemotherapy and HSC transplantation.
“We have developed an in vivo HSC transduction approach that requires only intravenous injections and could be provided as an outpatient treatment,” said Dr. Andre Lieber, MD, PhD, a professor of Medical Genetics at the University of Washington, and one of the two lab leaders driving the study. The secret to this approach? Helper-dependent adenovirus vectors (HDAds). These man-made gene-delivery tools replicate the capsid, or shell, of a virus and pack it with a payload of therapeutic genes. “HSCs are mobilized from the bone marrow into the peripheral blood stream and transduced with [HDAds] that target receptors present on primitive HSCs,” Dr. Lieber explained. “HSCs transduced in the periphery return to the bone marrow, persist there long-term, and contribute to all blood cell lineages.” Previously, the Lieber and Kiem groups had reported that this method worked well in mice. However, for this publication they wanted to test the method in a more relevant model. “The physiological similarities of the human and macaque hematopoietic systems make rhesus macaques (Macaca mulatta) a better model for assessing the safety and efficacy of our in vivo HSC gene therapy approach,” said Dr. Lieber….