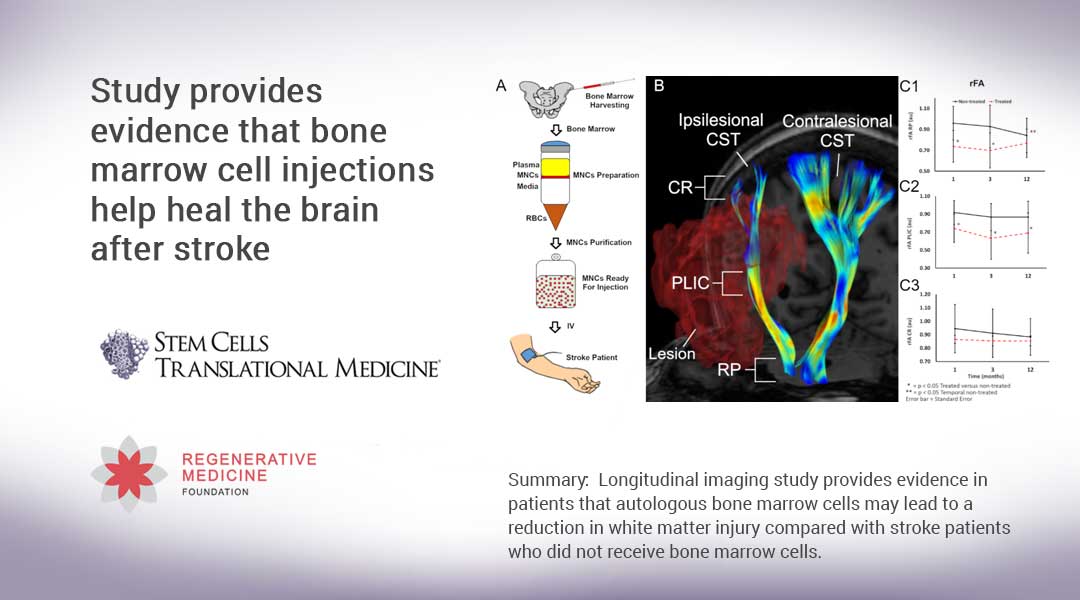
Summary: Longitudinal imaging study provides evidence in patients that autologous bone marrow cells may lead to a reduction in white matter injury compared with stroke patients who did not receive bone marrow cells.
Durham, NC – Results of a clinical trial released in STEM CELLS Translational Medicine provide evidence that treating patients with an injection of bone marrow cells may lead to a reduction in brain injury after a stroke.
The study was conducted by Muhammad E. Haque, Ph.D., Sean I. Savitz, M.D., and colleagues from the Institute for Stroke and Cerebrovascular Disease at The University of Texas Health Science Center in Houston. “Nearly 90 percent of patients who suffer an ischemic stroke – the most common type of stroke – exhibit weakness or paralysis to one side of the body,” Dr. Haque said. “Injuries to the corticospinal tract (CST), which is the main white matter connection in the brain responsible for carrying movement-related information to the spinal cord, is the primary cause of this motor function impairment. In stroke animal models, we’ve seen how bone marrow mononuclear cells (BM-MNC) attenuate secondary degeneration and enhance recovery, including white matter tract remodeling. That led us to our current study.”
Previously the team led by Dr. Savitz had conducted a phase I clinical trial on the safety and feasibility of intravenous administration of autologous (a patient’s own) bone marrow-derived mononuclear cells (BM-MNCs) in patients with ischemic stroke. In that study, they reported preliminary CST recovery in the part of the brainstem called the rostral pons. In their current work, they delved deeper into this intriguing finding by using 3D anatomical and DTI images obtained from MRI scans to compare extensive longitudinal microstructural changes in the white matter of BM-MNC-treated stroke patients to a group who did not receive the cells. (DTI – or diffusion tensor imaging – is an MRI technique that is most commonly used to examine the brain and estimate its white matter organization.)
The 37 patients in the study ranged in age from 18 to 80. While all received the standard stroke treatment and rehabilitation follow-up, 17 patients whose strokes were the most severe received the additional BM-MNC injections. Three months later, MRI scans of each patient showed, as the researchers expected, a decrease in the integrity of their CST. However, scans taken 12 months after the stroke occurred showed an improvement in the CST of the 17 patients who received injections. Conversely, the CST of the non-injected group exhibited ongoing and continuing microstructural injury and axonal degeneration.
“These results suggest the possibility of microstructural stabilization in the cell-injected group as compared with the non-treated patients,” Dr. Haque said, “We envision that future clinical trials might be directed toward identifying white matter protection or repair as an important mechanistic target of efficacy studies and potency assays for bone marrow cell therapies.”
“These clinical trial results are certainly encouraging and demonstrate the need to further pursue the use of cell-based regenerative therapies,” said Anthony Atala, M.D., Editor-in-Chief of STEM CELLS Translational Medicine and director of the Wake Forest Institute for Regenerative Medicine. “These outcomes suggest a potential approach that could change the brain health for millions of patients who suffer a stroke.”
###
The full article, “Longitudinal Neuroimaging Evaluation of the Cortical Spinal Tract in Stroke Patients Treated with Autologous Bone Marrow Cells,” can be accessed at https://stemcellsjournals.onlinelibrary.wiley.com/doi/abs/10.1002/sctm.20-0369.
Contact: Amy Laukka , Senior Media Relations Specialist, UTHealth
Office: 713-500-3030
E-mail: [email protected]
About STEM CELLS Translational Medicine: STEM CELLS Translational Medicine (SCTM), co-published by AlphaMed Press and Wiley, is a monthly peer-reviewed publication dedicated to significantly advancing the clinical utilization of stem cell molecular and cellular biology. By bridging stem cell research and clinical trials, SCTM will help move applications of these critical investigations closer to accepted best practices. SCTM is the official journal partner of Regenerative Medicine Foundation.
About AlphaMed Press: Established in 1983, AlphaMed Press with offices in Durham, NC, San Francisco, CA, and Belfast, Northern Ireland, publishes two other internationally renowned peer-reviewed journals: STEM CELLS® (http://www.StemCells.com), celebrating its 39th year, is the world’s first journal devoted to this fast paced field of research. The Oncologist® (http://www.TheOncologist.com), also a monthly peer-reviewed publication, entering its 26th year, is devoted to community and hospital-based oncologists and physicians entrusted with cancer patient care. All three journals are premier periodicals with globally recognized editorial boards dedicated to advancing knowledge and education in their focused disciplines.
About Wiley: Wiley, a global company, helps people and organizations develop the skills and knowledge they need to succeed. Our online scientific, technical, medical and scholarly journals, combined with our digital learning, assessment and certification solutions, help universities, learned societies, businesses, governments and individuals increase the academic and professional impact of their work. For more than 200 years, we have delivered consistent performance to our stakeholders. The company’s website can be accessed at http://www.wiley.com.
About Regenerative Medicine Foundation (RMF): The non-profit Regenerative Medicine Foundation fosters strategic collaborations to accelerate the development of regenerative medicine to improve health and deliver cures. RMF pursues its mission by producing its flagship World Stem Cell Summit, honouring leaders through the Stem Cell and Regenerative Medicine Action Awards, and promoting educational initiatives.