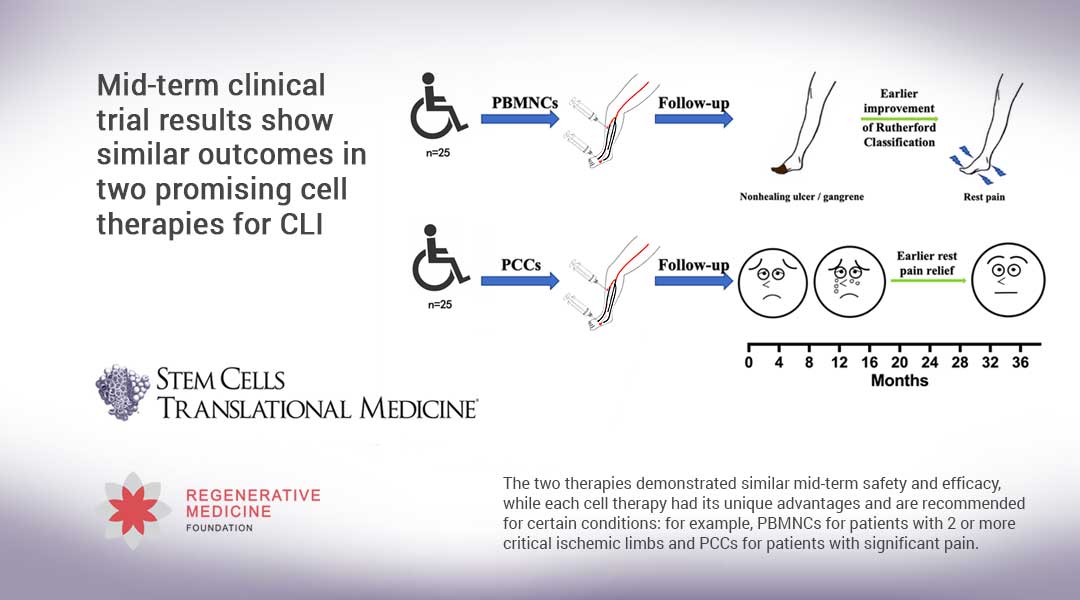
Durham, NC – Mid-term results of the first clinical trial designed specifically to evaluate the safety and effectiveness of two cell therapies that are showing early promise in treating angiitis-induced critical limb ischemia were released today in STEM CELLS Translational Medicine. The study, by researchers at Zhongshan Hospital/Fudan University in Shanghai, compared how transplantation of peripheral blood mononuclear cells fared versus transplantation of purified CD34+ cells in treating this condition.
It revealed both therapies yielded satisfactory results and provided evidence for more precise application of cell therapy under different conditions, the researchers say.
Critical limb ischemia (CLI) is a type of peripheral artery disease caused by severe blockage in the arteries of the lower extremities, which markedly reduces blood-flow. Angiitis-induced CLI (AICLI) occurs when the CLI is the result of inflammation of the walls of small blood vessels. This chronic condition results in severe pain in the feet or toes, even while resting. Left untreated, the complications of CLI often result in amputation.
“Cell transplantation, such as purified CD34+ cells (PCCs), which are multipotent hematopoietic stem cells, and peripheral blood mononuclear cells (PBMNCs), which are a mixture of highly specialized immune cells including T cells, NK cells and more, is gradually being used as a promising treatment for AICLI,” explained Zhihui Dong, M.D, who along with Weiguo Fu, M.D, led the investigation. “The aim of our trial was to compare the mid-term safety and efficacy between the two groups and determine their respective advantages.”
From April 2014 to September 2019, 50 AICLI patients were equally allocated to the two treatment groups. At the 36-month follow-up, 47 patients remained. The endpoints assessed were major amputation-free survival and total amputation-free survival. At six months after treatment these had been 96 percent and 84 percent in the PBMNCs group, and 96 percent and 72 percent in the PCCs group, respectively – rates that remained stable at the 12- and 24-month periods, too. The mid-term trial period focused on in this report – 36 months – once again confirmed stability.
“The PCCs group had a significant higher probability of earlier rest pain relief than the PBMNCs group, while earlier significant improvements in the Rutherford classification (which describes seven stages of peripheral artery disease) were observed in the PBMNCs group. Accordingly, PCCs would be preferred for patients with significant pain, while PBMNCs may be a good option for patients with two or more critically ischemic limbs,” Dr. Fu noted.
Additionally the study compared the therapies’ cost effectiveness and found that PCCs treatment was less cost-effective compared to PBMNCs treatment, mainly due to the extra cost of the purification process. “This finding is interesting and useful for future clinical trials in which we could consider avoiding purifying PBMNC into PCCs, thus reducing the cost of trials,” Dr. Dong said. “The next step should be to verify our outcomes in long-term trials involving larger numbers of patients.”
“The outcomes provided in this study of two cell therapies for the treatment AICLI show both to be effective and safe,” said Anthony Atala, MD, Editor-in-Chief of STEM CELLS Translational Medicine and director of the Wake Forest Institute for Regenerative Medicine. “This finding is promising and useful for the planning of future clinical trials.”
###
The full article, “Three-year outcomes of PBMNCs versus PCCs in the treatment of angiitis-induced no-option critical limb ischemia and a cost-effectiveness assessment,” can be accessed at https://stemcellsjournals.onlinelibrary.wiley.com/doi/abs/10.1002/sctm.20-0033.
About STEM CELLS Translational Medicine: STEM CELLS Translational Medicine (SCTM), co-published by AlphaMed Press and Wiley, is a monthly peer-reviewed publication dedicated to significantly advancing the clinical utilization of stem cell molecular and cellular biology. By bridging stem cell research and clinical trials, SCTM will help move applications of these critical investigations closer to accepted best practices. SCTM is the official journal partner of Regenerative Medicine Foundation.
About AlphaMed Press: Established in 1983, AlphaMed Press with offices in Durham, NC, San Francisco, CA, and Belfast, Northern Ireland, publishes two other internationally renowned peer-reviewed journals: STEM CELLS® (http://www.StemCells.com), celebrating its 39th year, is the world’s first journal devoted to this fast paced field of research. The Oncologist® (http://www.TheOncologist.com), also a monthly peer-reviewed publication, entering its 26th year, is devoted to community and hospital-based oncologists and physicians entrusted with cancer patient care. All three journals are premier periodicals with globally recognized editorial boards dedicated to advancing knowledge and education in their focused disciplines.
About Wiley: Wiley, a global company, helps people and organizations develop the skills and knowledge they need to succeed. Our online scientific, technical, medical and scholarly journals, combined with our digital learning, assessment and certification solutions, help universities, learned societies, businesses, governments and individuals increase the academic and professional impact of their work. For more than 200 years, we have delivered consistent performance to our stakeholders. The company’s website can be accessed at http://www.wiley.com.
About Regenerative Medicine Foundation (RMF): The non-profit Regenerative Medicine Foundation fosters strategic collaborations to accelerate the development of regenerative medicine to improve health and deliver cures. RMF pursues its mission by producing its flagship World Stem Cell Summit, honoring leaders through the Stem Cell and Regenerative Medicine Action Awards, and promoting educational initiatives