Study published in Nature Communications
By University of Miami Health System, Miller School of Medicine – Contact patient services
Miami, FL – July 8, 2020 — (Newswise) Scientists focused on finding better treatments or cures for types 1 or 2 diabetes are painfully aware of current limitations, including having to use animal tissue in studies that often don’t translate to human trials.
New research published June 29 in Nature Communications could help researchers overcome some of the biggest challenges of taking diabetes research from the lab to human trials and the clinic.
By using a technology first developed at the University of Miami Miller School of Medicine along with a Miller School patented approach to enhance the oxygenation of cultured tissues, researchers will likely be able to conduct real-time regeneration and development studies in the human pancreas.
The finding could lead to treatments that regenerate one’s own pancreas without the need for transplantation, according to the study’s senior author Juan Domínguez-Bendala, Ph.D., director of stem cell development for translational research and associate professor of Surgery at the Diabetes Research Institute, University of Miami Miller School of Medicine.
Dr. Bendala explained that in people who have type 1 diabetes, the body’s own immune system kills beta cells, or islet cells, in the pancreas that make insulin. Doctors have for years transplanted donor islet cells to replenish those cells.
But there are challenges to the approach. One is a scarcity of donors for organ transplantation. Another is when transplanting the islet cells is possible, the recipient’s body will likely reject the donor cells unless the recipient is immunosuppressed. Immunosuppression, alone, leads to complications.
“The two pillars of our research are to replenish the islet cells that have been lost and then to stop autoimmunity, which is the underlying cause of the disease,” Dr. Bendala said. “We also are interested in using endogenous regeneration. We have found that there are pancreatic stem cells that we call progenitors because they already have committed to become part of the pancreatic tissue. Ultimately, we want to induce them to replicate and give rise to new insulin-producing cells within the patient, instead of transplanting beta cells from an external source.”
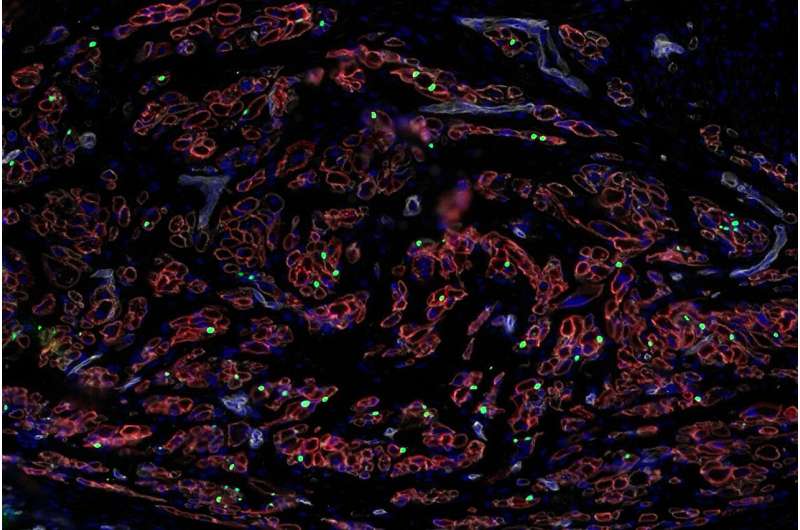
Human pancreatic slices are very thin slices of the pancreas that keep together the organ’s natural architecture, including the much-needed islets.
“The islets in these slices are surrounded by acinar cells, which make the digestive juices in the pancreas, and more importantly the ducts, where we have found the progenitor stem cells that can give rise to new beta cells,” Dr. Bendala said. “That’s why these slices are a very powerful tool to study the organ. It’s as if you had a window into the living pancreas.”
The problem when studying the regenerative process in human pancreatic slices has been that the tissue lasts only a couple of days before disintegrating and dying.
Dr. Bendala and colleagues determined that the main reason for cell death in the slices was a lack of oxygen. The pancreas is a very vascularized organ, and slicing it cuts off its blood supply.
Dr. Bendala and coauthor on the Nature Communications paper Ricardo Pastori, Ph.D., research professor of medicine, immunology, and microbiology and the director of the Molecular Biology Laboratory at the Diabetes Research Institute, circumvented the problem by placing human pancreatic slices in a culture device they invented that uses a perfluorocarbon (PFC) membrane.
“PFC is a compound that is so rich in oxygen that you can breathe it in its liquid form,” Dr. Bendala said. “We have published on this device and shown that islets survive and function much better when we culture them on PFC. And when we differentiate stem cells into beta cells, the process occurs much more efficiently when you put them in PFC. It was no surprise that when we placed the human pancreatic slices into the PFC membrane that they survived and did much better than controls. We could keep them alive for about 2 weeks, some went as long as 3 weeks, and they were fully functional during that time.”
Keeping human pancreatic slices alive for that long is a major breakthrough in diabetes research, especially in the area of islet cell regeneration, he said.
“You need a model when you study regeneration. Traditionally we have used the mouse model, and, unfortunately what happens in mice in the lab often doesn’t pan out in humans,” Dr. Bendala said. “This work is revolutionary because using these human pancreatic slices we can witness and monitor regeneration in a human model that resembles a real organ. That was not possible before because the tissue simply didn’t live long enough.”
The Miller School researchers also tested a molecule called BMP-7, which they have shown in previous studies to act as fuel to stem cells. They showed in this paper that BMP-7 can induce proliferation of pancreatic progenitors in human pancreatic slices.
“When we added BMP-7 to human pancreatic slices, we could detect progenitor cells activating, proliferating and then giving rise to new beta cells. We could see that happening before our very eyes,” he said.
The fact that the study also included tissue from human type 2 and type 1 diabetic patients makes it more much more likely that the research will facilitate progress to human clinical trials.
“I took a step back when I saw this for the first time. This was a living human pancreatic slice from a patient who had passed 10 days ago,” he said. “I couldn’t help but think, imagine if we had done this in the patient if he or she was still alive? It’s really powerful.”
Dr. Bendala sent PFC-based dishes at no cost to several other centers conducting diabetes research, so they could study the approach and potentially replicate the findings. In the meantime, Dr. Bendala and Miller School colleagues are screening molecules other than BMP-7 to see if they have potential to create new beta cells by inducing progenitors or by inducing the replication of pre-existing beta cells.
The goal is to have a therapy to present to the FDA to produce beta cells within a few years.
“These technologies will greatly accelerate our ability to decide what is going to work in clinical trials,” he said.