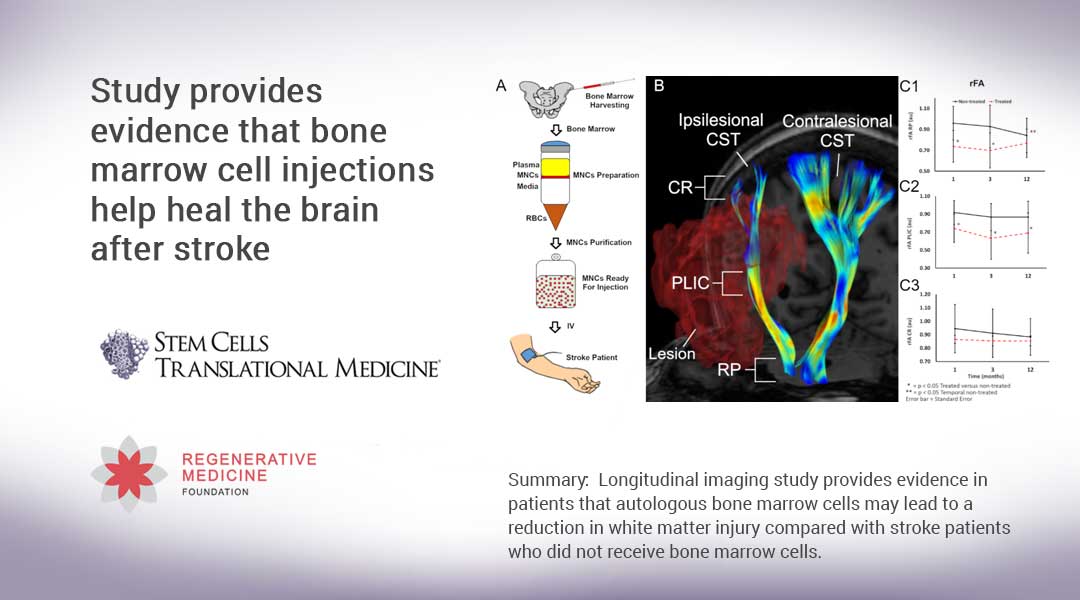
There is obviously a right way and a wrong way for clinicians to practice regenerative medicine in the clinic. AP reported this week that 12 patients in three states -Florida, Texas and Arizona- became infected after getting “stem cell” shots for joint and back pain and other problems.
The cell product culprit appears to be manufactured by an outfit called “Genetech” and was distributed by Liveyon, LLC and its associated clinics. Big mistake! Liveyon now is squarely in the bullseye of a very bad place, described by FDA Commissioner Scott Gottlieb as being a “bad actor”.
How are doctors to learn unbiased information on what is available in the “here and now”? How can patients and doctors understand the legal and ethical risks entailed? Only by rigorous self-education and awareness.
These topics are robustly covered in the upcoming World Stem Cell Summit, in Miami, Florida, January 22-25. There you will hear directly from Dr. Peter Marks, Director of the FDA Center for Biologics Evaluation and Research (CBER).
Also, there will be a showcase session designed to answer many of the burning questions- “Bringing order to the Wild West! Regulatory enforcement, lawsuits and ethical considerations for stem cells in clinical practice.” Review the full agenda and register for the summit.